Imagine being able to “correct” a gene responsible for disease just as you’d correct a typo in a document, and that single adjustment could cure a human condition once and for all. That is the true promise of CRISPR gene editing. Adapted from a bacterial immune mechanism, CRISPR (so named for its Clustered Regularly Interspaced Short Palindromic Repeats) allows DNA to be precisely altered inside living cells. Researchers can excise, erase, add, or replace individual DNA letters. From curing genetic diseases to enhancing food crops, this is no longer confined to science fiction; it is a powerful scientific reality that is defining our future.
What Is CRISPR
In 2013, two well-known biochemists published a paper declaring they’d discovered a conceivably revolutionary way of manipulating genes. CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, is an innovative technology that essentially takes advantage of the adaptive immune responses of bacteria. Discovered as a bacterial viral defense mechanism, CRISPR allows organisms to store pieces of viral genetic material, which subsequently directs RNA-guided endonucleases (including Cas9) when transcribed into RNA, to target and snip specific DNA sequences. The editing of the genetic code has become possible by utilizing this natural process, leading to the emergence of gene editing, which can make highly specific modifications in the genetic structure of the organism.

How CRISPR Works
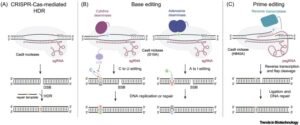
CRISPR–Cas9 Components
It consists of two principal components, one being a guide RNA (gRNA) and another being an enzyme called Cas9. The guide RNA, called gRNA, is programmed to recognize a given DNA sequence. Cas9 binds to the gRNA and searches the genome to locate the corresponding place, and at this location, it produces a clean cut. This is programmable and resembles GPS-guided molecular scissors.
Scientists Jennifer Doudna and Emmanuelle Charpentier got the Nobel Prize in Chemistry in 2020 for their research on making this precision genome-editing technology.
Guide RNA & DNA Repair
The repair machinery of the cell is set into motion after the cut. Researchers have a chance to manipulate how the repair is achieved:
- Knock-out: Excluding a collection of genes
- Knockin: Addition of new DNA
- Correction: correction of a one-letter mistake (base)
It then enables the removal of harmful mutations as well as the insertion of healthy versions of genes or the editing of the occurrence of a single-letter typo in a sentence, effectively.
Medical Applications of CRISPR Gene Editing
Here are the medical applications of CRISPR gene editing:
Sickle Cell & Beta‑Thalassemia
Casgevy (exagamglogene autotemcel) was approved as the first CRISPR-based medicine to treat sickle-cell disease and beta-thalassemia, two blood diseases passed through a person in December 2023. During therapy, the patient has their stem cells edited ex vivo so that he or she can develop a safe version of hemoglobin known as fetal hemoglobin. Patients in the clinical trials encountered more than 90 percent fewer painful crises and a vastly enhanced quality of life. Victoria Gray, who was one of the first patients, has not experienced symptoms anymore- this is evidence of the real-life impact.
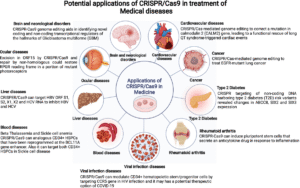
In Vivo Editing for Rare Diseases
In 2025, a newborn baby, KJ, with a rare liver disease (CPS1 deficiency) was supplied with a personalized base-editing CRISPR treatment, which was introduced directly into his liver with the help of lipid nanoparticle carriers. His ammonia went to norma,l and his symptoms improved within a short period- the first instance of in vivo correction of a deleterious gene in a human patient.
Cancer, Eye & Infectious Disease Treatments
CAR-T CRISPR technologies can better recognize and kill cancer cells by altering the immune T-cells during the treatment of lymphomas and leukemia.
EDIT-101 is a CRISPR therapy for Leber congenital amaurosis that is being tested in eye disease, as a direct retina application. Initial trial outcomes have been good safety and vision improvements.
CRISPR-based diagnostic tools such as DETECTR and SHERLOCK are Cas variants that detect the virus (Dengue, Zika, HIV, TB) much faster and in bedside conditions (particularly useful in low-resource settings).
The use of CRISPR to treat cystic fibrosis, Duchenne muscular dystrophy, blindness in inherited cases, and many other conditions, reports Reddit users, as experiments conducted before the steps of clinical practice have already been shown to be productive.
Novel Innovations: Base Editing & Prime Editing
New CRISPR technologies, such as base editing (BE) and prime editing (PE), equip us with greater specificity at a reduced risk:
- Base editing has the potential to replace one letter in the genetic code (e.g., A → G) without trimming of both strands. It is perfect in correcting disorders that involve a single letter, such as the sickle-cell mutation.
- Prime editing is a search-and-replace technology that uses Cas9 along with reverse transcriptase under the direction of a pegRNA. It enables homologous recombination and the insertion, deletion, or fixing of up to 89 percent of known disease-causing mutations without causing double-strand breaks.
These methods have already reversed progeria-like conditions in mice and increased life span, with potential applications to thousands of genetic illnesses. They are, however, associated with risks such as off-target editing when overexpressed or when improperly delivered; ongoing research is attempting to mitigate this.
Ethical, Regulatory & Fair Access Considerations
The He Jiankui Case
In 2018, Chinese researcher He Jiankui used an embryo editing technique to update the CCR5 gene to provide HIV-immune twins, a move not yet reviewed by an ethical review or consent process. The results were the arrest and imprisonment of a worldwide backlash. The case has turned into an effective caution against premature germline editing.
Oversight, Consent & Cost Equity
Germline editing is the process of modifying germline cells, capable of being passed on to other generations, which is prohibited or tightly regulated by various states. Ethical principles focus on the importance of telling people about the research, interaction with the population, and international collaboration. Issues such as designer babies, the disadvantaging of populations, and biospheric effects (such as irreversible modification of ecosystems by technologies like gene drives) are of concern.
Furthermore, CRISPR therapies such as Casgevy are almost USD 2 million a patient which is unsustainable in low-income environments. Actions are being taken to lower the prices and promote equitable access, particularly in those nations where genetic diseases are prevalent.
CRISPR Gene Editing: Pros and Cons
CRISPR technology has transformed genetic engineering providing lots of benefits; indeed there are a number of challenges that accompany the use of the technology. The points below present the most important advantages and disadvantages of CRISPR gene editing.
Pros
- Precision: CRISPR enables very specific modifications, with the ability to target specific strands of DNA with minimal interference to adjacent genes. This accuracy is essential when the intended genetic changes are put into practical use such as in treatment.
- Cost-Effectiveness: CRISPR is simpler and cheaper to design than CRISPR-based proteins, which makes gRNA more affordable to implement in research than older methods.
- Versatility: CRISPR has a wide range of applicability in fields and organisms and can be used in medicine, agriculture, and biotechnology to drive innovations such as gene therapies and crop enhancement.
- Rapid Development: CRISPR’s ease of design and implementation speeds up research and development cycles, enabling quicker scientific discoveries and findings.
- Prospect of New Uses: CRISPR also invites novel applications like synthetic biology, where new traits can be developed in organisms to resolve urgent issues in health and sustainability.
Cons
- Off-Target Effects: Although CRISPR is correct and accurate, it has the potential to cause off-target effects where the system affects undesired areas of the genome. This results in unpredictable consequences which cause deleterious mutations.
- Ethical Concerns: The capacity to modify the germline opens up some ethical issues, especially about human enhancement, designer babies, and possible undesirable societal impacts.
- Delivery Challenges: This problem is how effectively the CRISPR components (e.g., Cas9 and gRNA) can be delivered into target cells, especially in vivo, which severely restricts its applications to therapeutic uses.
- Regulatory Issues: Being a young technology, regulatory frameworks are still developing, and that introduces uncertainty when CRISPR-based therapies and products have to be approved and used.
- Misinformation and Personal Feelings: Any misunderstandings regarding CRISPR and its potential problems can cause resistance and fear in the population, which can hamper further research and research approvals.
Current Regulatory Approaches to CRISPR in Different Regions
There is a considerable regional difference in the regulations of CRISPR technology. Part of the major approaches is:
| Region | Regulatory Approach |
| United States | FDA considers CRISPR-Cas9 a gene therapy |
| European Union | EU subjects CRISPR-Cas9 to the GMO Directive regulation |
| China | China has a mixed CRISPR-Cas9 regulatory landscape, as there are several agencies involved |
Future Directions for CRISPR Research and Its Applications
CRISPR research and application have a dynamic future. There are some of the major areas:
- Medical use: CRISPR-Cas9 is under development to be used in a variety of medical applications, such as gene therapy and cancer treatment.
- Synthetic biology: CRISPRCas9 is also being applied to add new biological pathways and circuits into cells, to make new compounds and biofuels.
- Agriculture: CRISPR-Cas9 is being used in agriculture to grow higher yields and to add desirable features to crops.
The application of the CRISPR technology can transform a variety of avenues, including but not limited to the aspects of gene therapy and synthetic biology. Nevertheless, it equally presents serious ethical questions and dilemmas. When CRISPR develops technologies, it is important that we can have a nuanced discussion and regulation of the technology so that these technologies can be developed and used responsibly.
Conclusion
CRISPR gene editing is the new technology that can make specific targeted changes in DNA in a programmable manner. It has already delivered life-saving medicine, transformed diagnostics, and started reconstructing agriculture and biotech. New technologies, such as base editing and prime editing, are safer and precise. However, the key to its future success is considered to be solving the technical risk, ethical risk, and equity of access. CRISPR has the potential to save lives, advance global food security, and provide sustainable methods of solving global situation,s at the same time motivating a new generation of scientists through responsible research projects.
FAQs
What is CRISPR gene editing?
It is an instrument that accurately cuts and edits DNA, using a guide RNA and Cas enzyme that enables deletion, repair or addition of genes.
Is CRISPR safe?
New techniques such as base editing and prime editing minimize risks by not requiring a cut on the double strand. Nonetheless, the off-target effects, immune reactions, and long-term effects are still to be watched.
What diseases are treated with CRISPR now?
Casgevy can be used in sickle-cell disease and beta-thalassemia. There is an ongoing trial of rare diseases such as progeria, heritable blindness, immune diseases, cancer, and disorders of the liver.
What is the application of CRISPR in agriculture?
CRISPR is enhancing crops to become resistant to drought, pests, and spoilage. It is also being employed to make livestock resistant to diseases and ensure food waste is reduced, increasing nutrition and efficiency.
Which ethical considerations are major?
Concerns include germline editing and consent to posterity, access equity in treatment, environmental effects of gene drives, and international governance to discourage abuse.